Help Center Article
Importing Coffee into the United States
If you are importing coffee into the United States, you will need to involve three government agencies: CBP, the FDA, and the USDA.
Importing Coffee into the United States
Coffee shipments into the US involve three government agencies: US Customs & Border Protection (CBP), Food and Drug Administration (FDA), and US Department of Agriculture (USDA). Listed below you will find information for each agency that will assist you in clearing your coffee shipment.
What Customs wants you to know
CBP’s “informed compliance” publication that they want every coffee importer to understand is a must-read.
Prior to shipping
Make sure the Country of Origin is clearly marked on each bag. The publication in the link above from CBP provides clear guidelines on marking and classification of coffee products.
An Importer Security Filing (ISF) must be filed no later than 48 hours to your shipment's estimated time of departure from the last foreign port.
Export Documents – Certificate of Origin
Country of Origin certificate is required by the ICO (International Coffee Organization). This is the supplier or shipper’s responsibility. For more information you can go to http://www.ico.org/
At time of entry
Whole beans are duty free under the classification (HS) code 0901, but a Merchandise Processing Fee & Harbor Maintenance Fee will apply.
- Merchandise Processing Fee is 0.3464% depending on the value of the goods (min: $25, max: $528.33).
- Harbor Maintenance Fee is .125% depending on the value of goods (no min/max)
A customs bond will be required to clear goods through CBP. If you don’t have a continuous bond, you may purchase one through Flexport. A new bond takes approximately 5 days to process through CBP.
Your CBP entry can be filed five days prior to your shipment’s arrival. However, your goods can not be cleared until the FDA & USDA inspections are completed.
Inspections (FDA & USDA)
Both the FDA and the USDA will inspect your shipment.
FDA will sample your shipment based on size. Below are the FDA guidelines.
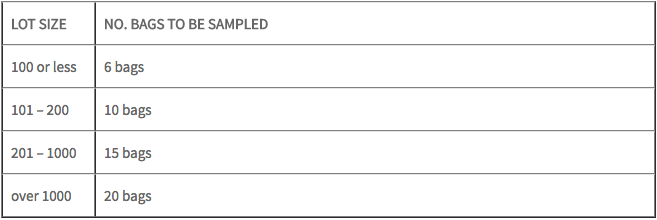
Inspections can result in additional charges if:
- A representative is required to accompany your shipment during inspection
- Inspection is not done in a timely manner and the delay incurs demurrage charges
- Additional paperwork is requested
Prior to arrival
USDA
The application for the permit to import plant or plant products (USDA PPQ587) must be completed and submitted to the USDA.
FDA
The FDA requires a Prior Notice Filing (which Flexport can help with) prior to your shipment’s arrival. You will need to provide the following information to your broker to assist them in completing the electronic filing:
- Name, business address, telephone, and email of the individual submitting Prior Notice, as well as the firm name and address (if applicable)
- Name, firm name (if applicable) and business address, telephone, and email of the individual transmitting Prior Notice (if someone else is transmitting Prior Notice on behalf of the submitter)
- Entry type and CBP identifier (if identifier is available). These include:
- FDA product code
- Common product name or market name
- Estimated quantity (from smallest package size to largest container)
- Lot, code number or other identifier (if food is required to have one)
- If the food is no longer in its natural state: manufacturer’s name and either 1) the registration number, city, and country of the manufacturer, or 2) both the full address of the manufacturer and the reason the registration number is not provided (reasons listed in the Compliance Policy Guide for Prior Notice of Imported Food)
- If the food is in its natural state: name of grower and growing location, if known
- FDA Country of Production
- Shipper’s (sender’s, if food is mailed) name and full address
- Country from which food is shipped; or, if food is imported by international mail, the anticipated date of mailing and country from which food is mailed
- Anticipated arrival information (location, date, and time); or, if food is imported by international mail, the U.S. recipient’s name and address
- Name and full address of importer, owner, and consignee, unless the shipment is imported or offered for import for transshipment through the U.S. under a transportation and exportation (T&E) entry; or, if food is imported by international mail, the U.S. recipient’s name and address
- Carrier and mode of transportation (except for food imported by international mail)
- Planned shipment information (except for food imported by international mail)
- Any country to which the article has been refused entry. (2011 IFR)
Registration
The FDA also requires registration for all food facilities. This applies to both US import and export facilities that handle and process coffee.
To register go to the following links on the FDA website:
Making sure your paperwork is in order prior to your shipment’s arrival will help to ensure your goods are cleared in a timely and cost-effective manner.
Note that Flexport does not import coffee at this time.
